Introduction
Classic Hodgkin Lymphoma (CHL) is currently classified into histological subtypes based on morphology and antigen marker expression. However, additional biological features might help guide treatment strategies and inform prognosis. Here, we aimed to uncover disease heterogeneity and biologic subtypes of CHL based on multi-dimensional molecular profiling ( Fig A) capturing somatic gene mutations, malignant cell expression phenotypes and altered tumor microenvironment (TME) architecture.
METHODS
We performed exome/targeted sequencing on flow-sorted or micro-dissected HRS cells from fresh-frozen CHL biopsies (n=116) of patients treated at BC Cancer. In addition, we constructed tissue microarrays (TMA) from this cohort on which we performed GeoMx® Whole Transcriptome Assay (WTA) to obtain gene expression profiles from CD30+ HRS cells and multiplexed imaging analyses using multi-colour immunofluorescence (mIF) to delineate the spatial TME ecosystem. Cox regression analysis was performed to identify outcome differences according to molecular features in CHL patients treated with ABVD-like chemotherapy (n=104).
Results
Mutational and copy number analyses identified known recurrent driver events including mutations and copy number changes in SOCS1, STAT6, TNFAIP3, B2M, REL and the PDL1/PDL2 locus. These mutational findings provided a statistically powered framework to investigate the association between genomic alterations with pathological and clinical disease parameters including outcomes. Mutations affecting BCL7A, TNFAIP3, and 2p15 (REL) amplifications were strongly enriched in EBV- CHL compared to EBV+ CHL. In line with “thymic mutations” reported in grey zone lymphoma, STAT6, GNA13, ITPKB, and TBL1XR1 mutations were significantly enriched in CHL with involvement of the anterior mediastinum. ZNF217, SPEN and CD58 mutations were significantly associated with progression-free survival (PFS) (all P < .01). When investigating outcome correlation according to age group, genomic alteration in STAT6 including both single nucleotide variants and STAT6 amplification, was the most significant feature associated with unfavorable PFS in patients <45 years of age ( P = .013).
Alterations in the antigen recognition system, including loss of major histocompatibility complex (MHC) is one of the most studied mechanisms of immune escape in CHL. Mutations in CSF2RB, GNA13, TNFAIP3, and STAT6 were significantly associated with MHC-I loss. Strikingly, all cases harboring CSF2RB mutations (n=23) also showed loss of MHC-I. GeoMx analysis revealed that IFN-a and IFN-g pathways were significantly upregulated in MHC-I+ HRS cells. Mutations in HIST1H1E and BCL7A were significantly enriched in cases with loss of both MHC-I and MHC-II expression.
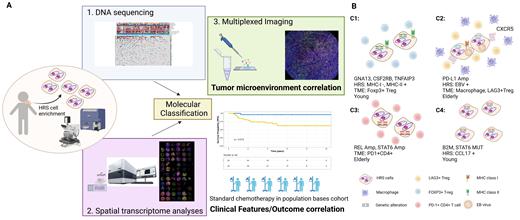
To define mutational subtypes of CHL, we next applied non-negative matrix factorization (NMF) consensus clustering and discovered four robust subsets of tumors (clusters) using recurrent genomic events. Cluster1 (C1) is characterized by mutations in TNFAIP3, CSF2RB and GNA13, younger age, loss of MHC-I, and up-regulation of GCB and fatty acid metabolism signatures. C2 was associated with old age at initial diagnosis, EBV positivity and significant upregulation of the IFN-g pathway. C3 is characterized by REL and STAT6 amplification andexpression of a DNA repair signature. C4 includes samples from mostly younger patients with STAT6 and B2M mutations and high expression of CCL17 by HRS cells. MIF analyses further identified correlations between each mutational NMF cluster and TME composition ( Fig B), and in particular specific CD4+ T cell subsets. The TME of tumors was significantly enriched by C1: FOXP3+Tregs, C2: LAG3+CD4+ cells, and C3: PD1+CD4+ cells. C4 tumors showed no positive correlation with any of these immune cell compartments. C2 tumors were also significantly associated with the abundance of CD68+ macrophages.
Conclusion
Our multi-dimensional profiling approach enabled us to delineate molecular profiles of HRS cells that are linked to distinct TME patterns. These linkages have implications for current pathogenesis models, molecular subtyping of CHL, and identification of cellular vulnerabilities that might be therapeutically exploitable via targeting of HRS cell phenotypes and/or immune escape mechanisms.
Disclosures
Sarkozy:AbbVie: Honoraria; BMS: Consultancy; Janssen: Consultancy; Incyte Bioscience: Consultancy, Other: Travel, Accommodations, Expenses; GSK: Consultancy; Gilead: Other: Congress fees; Roche: Other: Travel, Accommodations, Expenses, Research Funding; Prelude Therapeutics: Consultancy; Beigene: Consultancy; Lilly: Honoraria; Gilead: Other: Travel, Accommodations, Expenses; Takeda: Other: Travel, Accommodations, Expenses. Prica:Abbvie: Honoraria; Astra-Zeneca: Honoraria; Kite Gilead: Honoraria. Savage:BMS: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Seagen: Honoraria; Roche: Research Funding. Scott:Abbvie, AstraZeneca, Incyte: Consultancy; Janssen and Roche: Research Funding. Steidl:Seattle Genetics, AbbVie, and Bayer: Consultancy; Bristol Myers Squibb, Epizyme and Trillium Therapeutics Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal